4 skin conditions caused by Covid-19 vaccine
COVID-19 vaccine and skin rash
COVID-19 pandemic surprised us on many fronts; new symptoms, unexpected disease outcomes and mutations, just to name a few. The subsequent rapid development of COVID-19 vaccine gave the world relief and hope. However, this relief and hope was followed by many questions and concerns about the unknowns of the vaccine.
In order to explain the potential skin reactions to COVID-19 vaccine, let’s start with understanding the COVID-19 vaccine itself.
What are the main types of COVID-19 vaccines?
Four main types of COVID-19 vaccines received authorization and approval for use. These are; nucleic acid-based vaccine (mRNA and DNA vaccines), viral vector vaccine, protein subunit vaccine and inactivated vaccine (1,2). Based on the Our World in Data project, two messenger RNA (mRNA) vaccines by Pfizer-BioNTech and Moderna, are most preferred ones in the US and European Union (3).
The type of authorized vaccine/s against COVID-19 varies among countries. In addition, some of them were suspended or restricted for use over concerns about possible side effects.
Spike protein and adjuvant are the two key components of most Covid vaccines.
What is a Spike protein ?
Spike protein, also known as S protein, is found on the outer surface of the virus in the form of a protrusion and helps viral entry into human cells. There are around 30 spike proteins on the surface of each SARS-CoV-2 viral particle (4). Studies show that there is a high similarity between certain primary structures of human proteins and the S protein (5), (6). Due to these findings, it is assumed that the immune system, while attempting to fight with the virus itself, may be attacking its own healthy cells as well.
What is an adjuvant ?
Adjuvants are molecules that are administered together with the vaccine, to boost immune responses.
Nucleic acid-based vaccine
A nucleic acid vaccine delivers a set of instructions to our cells for them to make the specific fragments of the virus (in this case S protein), that we want our immune system to recognize and generate an immune response. Upon exposure to COVID-19 virus, the immune cells will recognize the S protein of the virus and they will be ready to fight it off.
mRNA molecules cannot get into the cells on their own, therefore they are packaged in a polyethylene glycol (PEG) – 2000 containing lipid nanoparticles. PEGs are already being used in daily products such as toothpastes, fabric softeners and moisturizers. They are also found in numerous medications. However, it is the first time that they have been used in an approved vaccine and delivered intramuscularly. Although rare, PEG induced local and systemic allergic reactions have been reported (7,8, 9, 10).
Viral vector vaccine
Instructions (genetic code) for making S protein are inserted into a harmless virus, so called a vector virus. When the vector virus is injected to the muscle cells, S protein is produced in small amounts. The body is tricked into being infected and the immune system learns to recognize and attack S protein containing COVID-19 virus (11). This is a relatively new technology and its authorization for use in humans is currently restricted to specific types of COVID-19 and Ebola vaccines only.
Oxford-AstraZeneca, Janssen (Johnson & Johnson) and Sputnik V are among the recently developed COVID-19 viral vector vaccines, which have received emergency use authorization in various countries around the world.
Inactivated virus vaccine
An inactivated (dead) virus means the virus cannot replicate and cause disease, but when injected, it can still be recognized as a foreign substance to generate an immune response. This approach often requires addition of an adjuvant and it has been successfully employed for many decades for vaccines such as hepatitis A vaccine, poliovirus vaccine and whole cell pertussis (whooping cough) vaccine. Some of the vaccines developed against COVID-19, eg. by Sinovac, Sinopharm BIBP and Covaxin also use this technology. In order to stimulate the immune response, all three vaccines include an aluminum based compound as an adjuvant.
Protein subunit vaccine
A protein subunit vaccine delivers only parts of the virus (in this case synthetically produced S protein), which are produced outside the body and delivered with an injection. Once the immune cells learn to recognize these subunits, they will also be ready to detect them upon exposure to COVID-19 virus itself. Nuvaxoid (Novavax) vaccine utilizes this technology and delivers S protein with a saponin-adjuvant called Matrix-M™. Although use of Matrix-M™ in humans is being evaluated in several ongoing clinical trials, currently it has only received authorization for human use as an adjuvant for the vaccine by Novavax.
Can COVID-19 vaccine cause skin reactions ?
COVID-19 vaccine may induce skin rashes, blisters, swelling, itch and/or tenderness. These reactions may be local – at the site of injection, distant from the site of injection or they can involve the entire skin surface. Contrary to the common perception, skin reactions may not be immediate and the interval between the vaccination and the onset of skin reactions may be longer than a week (10, 12). It is also not uncommon that a skin reaction occurs after both vaccine doses, only after the second dose or only after the booster.
Redness, induration, swelling and tenderness may appear at or near the site of injection. Injection site/delayed local reactions (also known as COVID-arm) are the most frequently encountered side effects, the latter may present several days after, and often self-resolves (12, 13). Depending on severity, cool compresses, topical steroids and oral histamines may be recommended.
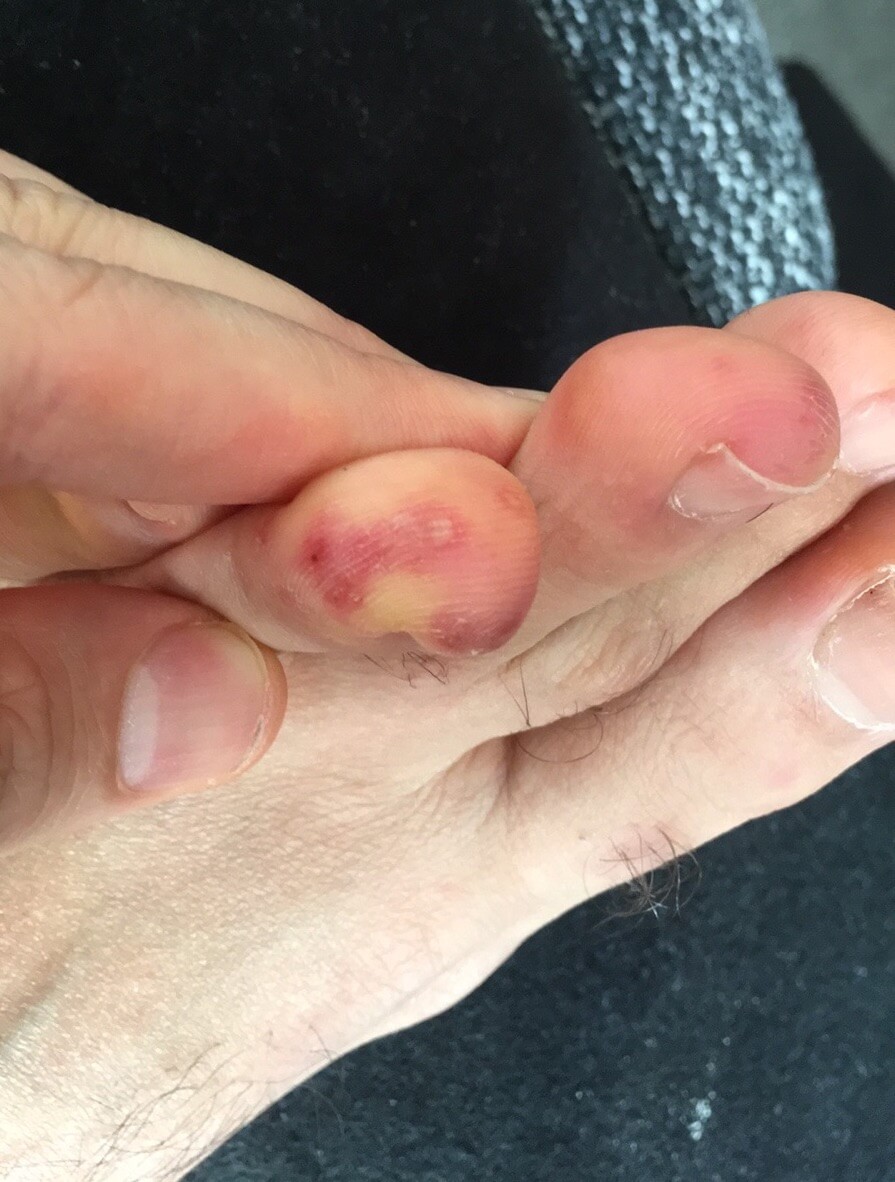
Pernio/Chilbain-like lesions (also known as COVID-toes) are red to purple colored painless patches on the hands and feet. The duration of these lesions is usually less than a month and they typically heal without a treatment. Occasionally topical steroids may be recommended. Pernio-like lesions were initially observed after COVID-19 infection. However, recent reports have shown that COVID-19 vaccine also caused similar lesions (10, 15). Little is known about the effects of S protein or its subunits that are found both in the vaccine (in lesser amounts) and the virus itself (in higher amounts). There may be a possibility that these lesions caused both by the vaccine and the virus itself could be triggered by the body’s reaction to the S protein or its small fragments.
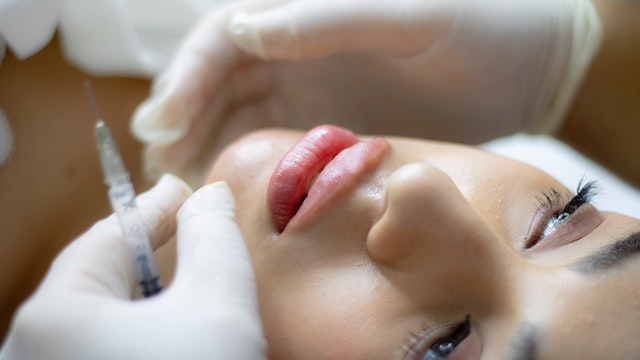
Filler reactions
Swelling at the site of cosmetic fillers has been reported both after a COVID-19 infection and after COVID-19 vaccine (14). In case of the vaccine, the reactions often developed within a day or two. These reactions can be resolved but a visit to a board certified dermatologist is required.
Morbilliform rash
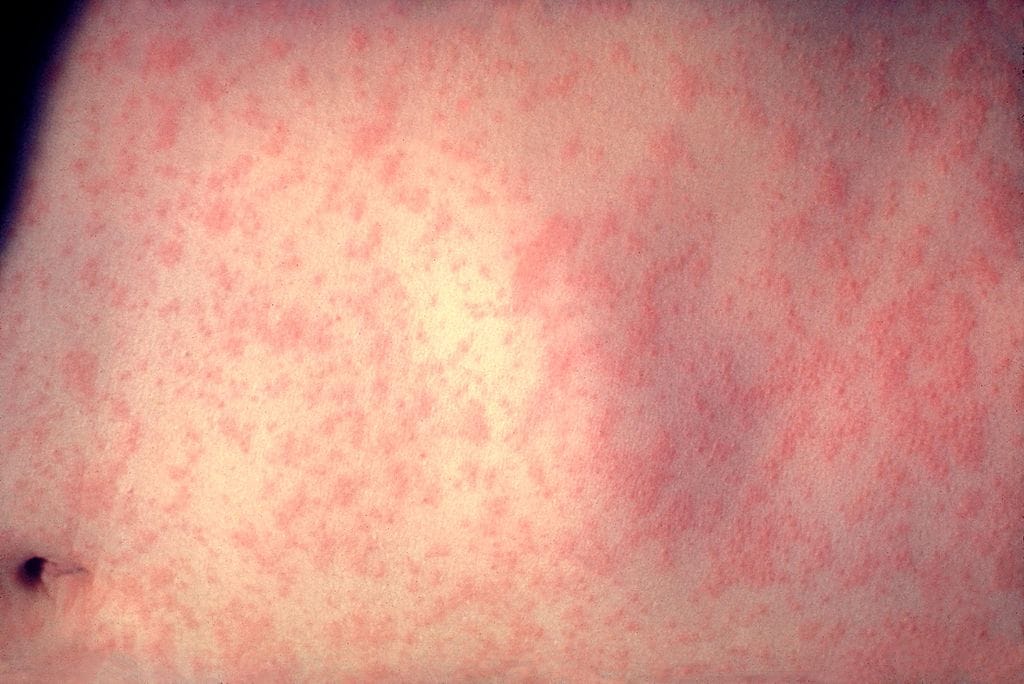
Morbilliform rashes generally present as pink-red flat or slightly raised lesions on the trunk, arms and/or legs. Similar to Chilbain-like lesions, these rashes were also observed both after COVID-19 infection and after COVID-19 vaccine. They generally appear a few days after the vaccine and disappear within a week. Topical corticosteroids and antihistamines may be recommended. When they occur within the 4 hours of vaccination and as a part of an anaphylactic reaction (accompanied by symptoms such as swelling, flushing, itching, dizziness, breathing difficulties, increase in heart rate, nausea and vomiting) an immediate medical care is required.
Urticaria
Urticaria (also known as hives or wheals) often appears as multiple, raised, skin colored to red, itchy lesions on the arms, trunk and/or legs. The length of time between the administration of the vaccine and the onset of urticaria may range from minutes to days (14, 15). If the lesions appear within the first 4 hours of vaccination, a medical evaluation is necessary (14). When accompanied by symptoms such as swelling, flushing, itching, dizziness, breathing difficulties, nausea and vomiting, immediate medical care is required.
Can skin reactions after the vaccine be life threatening ?
Although extremely rare, life-threatening allergic reactions to the COVID-19 vaccines have been reported. PEG-2000 (not confirmed but a potential allergen) contained in mRNA vaccines as well as Polysorbate 80 (chemically related to PEG) contained in vaccines such as AstraZeneca and Novavax may potentially trigger anaphylaxis. Moreover, especially in case of skin diseases, rashes caused by different types of diseases may look similar and thus make it difficult to assess severity. Therefore it is important to monitor any new symptoms after the vaccination and seek medical care when necessary. Most rashes that develop after the vaccine are not severe, generally resolve on their own and are not a contraindication for a second dose or a booster vaccine.
In conclusion
As mentioned earlier, some of the COVID-19 vaccines employ new technologies and/or adjuvants that are used in humans for the first time. Event monitoring of safety of COVID-19 vaccines still continues, and to date real-world data on skin reactions remain limited. Moreover, patients with pre-existing conditions requiring immunosuppressive medications were generally excluded from COVID-19 vaccine clinical trials; therefore, flares of pre-existing chronic inflammatory and autoimmune skin diseases might be underreported.
Nevertheless, although COVID-19 vaccine may carry the risk of causing skin reactions, an infection by the COVID-19 virus itself and the drugs used for managing the symptoms can lead to many serious conditions with detrimental effects on one’s health.
About the author
Pinar Avci received her MD and PhD degrees from Semmelweis University and completed a post-doctoral fellowship at Massachusetts General Hospital, Harvard Medical School. She is currently a dermatology resident at Ludwig Maximilian University of Munich. Her research focuses on utilization of imaging modalities such as confocal microscopy, multispectral cameras and artificial intelligence in dermatology.
Read more about Pinar Avci’s research work here
References
- https://www.immunology.org/coronavirus/connect-coronavirus-public-engagement-resources/types-vaccines-for-covid-19
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7553041/
- https://ourworldindata.org/covid-vaccinations
- https://pubmed.ncbi.nlm.nih.gov/32805734/
- https://www.nature.com/articles/d41586-021-00149-1
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7246018/
- https://pubmed.ncbi.nlm.nih.gov/33011299/
- https://accp1.onlinelibrary.wiley.com/doi/10.1002/jcph.1824
- https://pubmed.ncbi.nlm.nih.gov/33825239/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8470727/
- https://www.cell.com/trends/biochemical-sciences/fulltext/S0968-0004(21)00047-5
- https://jamanetwork.com/journals/jamadermatology/fullarticle/2779643
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8024548/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8165093/
- https://pubmed.ncbi.nlm.nih.gov/34661927/
- https://pubmed.ncbi.nlm.nih.gov/33560783/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8470727/
- https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained
Ask a Dermatologist
Anonymous, fast and secure!

The Specialist doctor from the University Hospital in Gothenburg, alumnus UC Berkeley. My doctoral dissertation is about Digital Health and I have published 5 scientific articles in teledermatology and artificial intelligence and others.
![Hives (Urticaria) (17) skin [ICD-10 L50]](https://firstderm.com/wp-content/uploads/Hives-Urticaria-17-skin-ICD-10-L50-e1592936953496.jpg)

